Background: Increasing evidence shows the impact of mutational burden in acute myeloid leukemia (AML) and impact on clinical response. Classifying these mutations into exclusive sub-types that are mutually exclusive was recently attempted. We sought to identify differences in mutational burden in AML patients based on race.
Methods: We retrospectively reviewed the patient charts to distinguish the mutational markers of AML that substantially impact the outcome of AML. We categorized the mutations in seven functional groups with mutually exclusive mutations Signaling and kinase pathway (FLT3, KRAS, NRAS, KIT, PNPN1, JAK2, CBL), Epigenetic modifiers (DNMT3A, IDH1, IDH2, TET2, ASXL1, EZH2, and MLL/KMT2A), Nucleophosmin (NPM1), Transcription factors (CEBPA, RUNX1, and GATA2), Tumor suppressors (TP53), Spliceosome complex (SRSF2, U2AF1, SF3B1, and ZRSR2), and Cohesin complex (RAD21, STAG1, STAG2, SMC1A, and SMC3). For estimating racial distribution, we included only Whites and African Americans (AA) in the study as they represent 95% of the total population at our Cancer Center. Remission and relapse were defined per standard guidelines. We compiled data of all newly diagnosed AML patients treated at our institution between 2016 to the end of 2019. Both next generation sequencing (NGS) and Polymerase Chain Reaction (PCR) methods of genetic marker recognition techniques were included in the study.
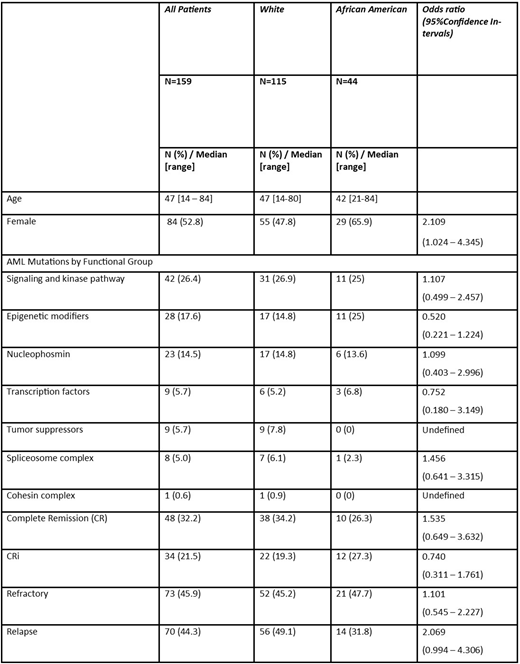
Results: 159 patients with AML were included in the analysis. We excluded seven patients of different race, including Asian (n=2), Hispanic (n=3), and unknown (n=2). The median age of the patients at diagnosis were 47 years (range 14 - 84 years), 73.3 % were white Caucasian, and 52.8% were female. The median age for white and African American (AA) patients was similar (47 vs 42 year respectively, p=0.55659), however, AAs have more female than Whites (65.9% vs. 47.8%, p=0.04164). In descriptive analysis of genetic marker mutation distributions between Whites and AA we observed signaling and kinase pathway 26.9% vs 25%, p=0.80231; epigenetic modifiers 14.8% vs 25%, p=0.13144; nucleophosmin 14.8% vs 13.6%, p=85460; transcription factors 5.2% vs 6.82%, p=0.69686; tumor suppressors 7.8% vs 0%; spliceosome complex 6.1% vs 2.3%, p=0.32647 and cohesin complex 1% vs 0%, respectively. Overall, 32.2% achieved complete remission (CR), 21.5% complete remission with incomplete hematologic recovery (CRi) and 45.6% Refractory. The CR + CRi rates of Whites and AA were not statistically significant (54.8% vs 52.3% respectively, p=0.77699). The median number of induction required for CR in both races was the same (2 and 2, respectively). We did not find any differences in number of induction for achieving CR by race. However, the rate of relapse was higher in white patients than in AA (49.1% vs 31.8%, respectively) (p=0.05039).
Conclusion: This analysis suggests that there might be variations in functional categories of mutations markers in AML patients by race, tumor suppressors (TP53) found more frequently in whites and epigenetic modifiers in AA. This might be at least in part the reason for a higher relapse rate among whites. Additional studies and larger cohorts are needed to further explore the correlation between race, molecular markers and outcomes for AML.
Cortes:Daiichi Sankyo:Consultancy, Research Funding;Astellas:Research Funding;BioPath Holdings:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;Takeda:Consultancy, Research Funding;Pfizer:Consultancy, Research Funding;Telios:Research Funding;Jazz Pharmaceuticals:Consultancy, Research Funding;Merus:Research Funding;Immunogen:Research Funding;BiolineRx:Consultancy, Research Funding;Bristol-Myers Squibb:Research Funding;Arog:Research Funding;Amphivena Therapeutics:Research Funding;Novartis:Consultancy, Research Funding;Sun Pharma:Research Funding.Kota:Novartis:Consultancy;Pfizer:Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal